On-chip Separation and Identification
Scientific Leader: Anne-Marie Haghiri
Principle Investigators: Isabelle Le Potier, Jean Gamby
Postdocs: François-Damien Delapierre
Ph.D students : Fatima Flores-Galicia, Thève Lok (with LadHyx, Ecole Polytechnique), Choayb Omar
Medical diagnosis has to be more and more precise and discriminant, especially for certain pathologies close or been similar requiring different therapeutic coverage. Current macroscopic methods based on chromatography techniques coupled to mass spectrometry remain long and tedious. The development of microfluidics bioanalysis becomes thus very important, since fluidic platforms could offer short time to result on volume lower than one microliter, low cost per test, multiplexing on several analytes and portability.
When the diagnosis is based on the separation of several biomarkers, among which the composition and the structure are close, the parameters governing the separation must be perfectly controlled. In this context severalinnovative fluidic devices such as the Flow Field Effect transistor (FFET) [Q. Zhang et al, Electrophoresis 38 (2017) 953-976] for separation of proteins have been studied [A-M. Haghiri-Gosnet et al, patent FR/5.09.14/ FR1458333-PCT/EP2015/070211]. This research has been supported by two Labex PUCEDIA and FLUTEC projects until 2018.
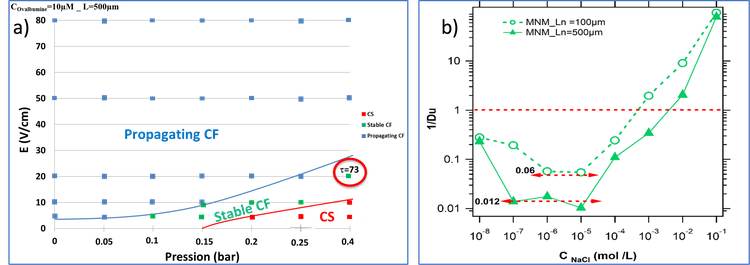
In parallel the detection of low abundance analytes in complex samples remains a real challenge. Concentration-polarization (CP) - based focusing electrokinetics nanofluidic devices have been developed in order to simultaneously detect and enrich highly diluted analytes on-a-chip in several minutes up to a factor 103. However, stabilization of focal points over long time under the application of the electric field remains as a technical bottleneck. Based on the double theoretical and experimental expertise of our group on the mechanisms of preconcentration in such a nanofluidic device, we have recently proposed a pressure-assisted preconcentration protocol in which a hydrodynamic counter-pressure P is added for a better control of the preconcentration frontline location. Figure 2a) shows a typical "electric field / pressure" diagram obtained for ovalbumin. The stabilization of the CF focusing regime by the additional pressure is obtained at low pressures and electric fields. Such diagram has also evidenced the role of the length of the nanoslit on the position of the focal points [Electropreconcentration diagrams to optimize molecular enrichment with low counter pressure in a nanofluidic device, Electrophoresis 41,18-19 (2020) 1617- 1626]. Conductance measurements have been also recorded for several nanofluidic devices with different nanoslit lengths. An eliminating procedure was proposed by subtracting the external microchannels conductances which highlights a net observation of the inverse Duhkin number prediction depending to the nanoslit length (Figure 2b) [Modeling the role played by nanoslit lengths on conductance changes into micro nano microfluidics devices, [Electrochimica Acta 374 (2021) 137930].
In parallel, we have also proposed a new nanofluidic geometry that integrates series of vertical nanochannels [A-M. Haghiri-Gosnet et al, FR1660855 - European PCT phase WO/2018/087240 - US2020/0061610A1 (27 feb 2020)] (Figure 2). Our experimental results obtained during DIFLUSEL ANR Astrid project have been selected by the French DGA as one of the best innovations of 2016. Coupled with the pressure-assisted preconcentration method, this new nanofluidic chip produces a fluorescence « barcode » as a specific signature of the target biomolecule. This “barcode” nanofluidic chip is under study in the context of the running NANOCODE ANR project. COMSOL simulations are under progress to predict the location of the focal fluorescent frontline as function of the geometry (channel length and width).
Our team is also involved in a research project focused on the development of a single-use integrated microfluidic platform allowing the detection of pathogens responsible for severe infections. The microfluidic platform integrates different modules allowing the extraction of the pathogens directly from the whole blood sample, the lysis of these pathogens and direct electrochemical detection without PCR amplification. These platforms will be used to analyze a panel of bacterial strains isolated on a broad spectrum of pathogens of septicemia and presenting different types of antibiotic resistance (coll. Pr E. Cambau, H. Jacquier, Lariboisière hospital, AP-HP).
Concerning electrochemistry on chip, the need to develop simple label-free DNA hybridization platforms for specific detection at extremely low concentration approaching the attomolar is becoming an important issue. We have shown that working under “fast” flow at a high Peclet number allows enhancing the number of captured molecules if both microelectrodes and fluidic channel have been well designed. The first demonstration has been done with the microfluidic-multiplexed platform that integrates carbon nanotubes associated with ferrocene for direct detection of pathogenic viral DNA from Hepatitis C and genomic DNA from Mycobacterium tuberculosis in clinical isolates [B. Zribi et al, Biomicrofluidics 10 (2016) 014115]. With the optimized fluidic protocol, the limit of detection (LOD) has been enhanced at the femtomolar level. Similarly, simulations of new microelectrode arrangements have been performed to stabilize the rest potential between a larger counter electrode (CE) and a chemically functionalized gold working electrode (WE) onto which an auto-assembled monolayer of thiolated-DNA probe was grafted (MC Horny PhD) [M-C. Horny et al, Lab on a Chip 16 (2016) 4373-4381]. In collaboration with A. Ouerghi (team MAT2D), we have also developed an electrochemical sensor with epitaxial graphene as working electrode. At the macroscale, a graphene bilayer exhibits a detection limit of 0.1 femtomolar. Since a perfect graphene basal plane has no electrochemical reactivity, 200nm-wide artificial edges controlled in density have been generated using nanoimprint lithography. Such nanoprinted graphene array functionalized with ferrocene can detect the presence of hepatitis C DNA strands, diluted to a concentration as low as 0.1 attomolar [Nanoscale 8 (2016) 15479 - 15485]. Integrated inside a fluidic chamber, detection below the attomolar regime is also possible. Charge transfer inside this "epitaxial graphene / ferrocene" platform was also studied by ARPES synchrotron measurements (at SOLEIL). We demonstrated the existence of a gap opening after ferrocene grafting associated with a p-doping of graphene. Electrochemical activity and charge transfer (Ks = 7s-1) increase thanks to the low coverage rate of the graft molecules and the small spacer (ethylene diamine). [Carbon 153 (2019) 557-564].